Why the FDA should grant an EUA for fluvoxamine
Why the FDA should grant an EUA for fluvoxamine immediately

Considering the wealth of data from cell, animal and clinical studies, there is now ample evidence that fluvoxamine is arguably the most effective COVID outpatient treatment and should be considered by the FDA for an EUA immediately because it will save hundreds of thousands of lives that are being needless lost today.
The argument is simple: every piece of evidence we have shows benefit >>>>risk and the risk of the drug is about comparable to taking a Tylenol for 14 days. Side effect profile is mild nausea in about 1% of patients. I have never heard of even a single case where the drug failed to recover a COVID patient, regardless of the initial state of the patient.
Imagine you are tasked with one goal: maximize the number of lives saved over the next 2 months. You are given just two options: a) tell people to keep doing what they've been doing while we wait for 2 months for the phase 3 data or b) give everyone a fluvoxamine prescription right now (based on the data we have now on the table).
The expected results based on the data on the table today, the Fisher Exact Test, and standard mathematical expectation calculations are:
a) 0 lives saved (following CDC advice); this is the baseline
b) >128,000 lives saved relative to baseline (95% confidence of effect size of 75% or more)
True doctors will always opt for a) because they are trained to never give a drug unless it passes a phase 3 trial since it may not be safe or effective.
But that is flawed logic. We are in a pandemic and this is a repurposed FDA-approved drug. That logic should not apply because it will not save lives.
With regards to safety, we are talking a repurposed drug here that has been used by tens of millions of people (especially those who are older and have many comorbidities) over the past 37 years (FDA approved for the last 27 years). And we know from the short and long term data from the phase 2 trial that there weren't any safety issues when the drug is used against COVID. There are no reported deaths from fluvoxamine overdose in the scientific literature. So safety isn't an issue since the placebo is killing people.
So now it's down to efficacy: can the drug make any difference in the death rate?
In just two trials, we have a p<.0001, an average effect size of 100%, and a 95% C.I. that the effect size is 75% or more. We'll get to the details in a minute. And there is a lot more than just the two trials... there are multiple observational studies showing very strong p values and effect sizes.
While the effect size could be zero, that seems very unlikely. For each of the 50 symptomatic patients in the Seftel study, each and every one resolved symptoms in an average or 3 days. That could happen because we got lucky, but it's unlikely.
At this point, it is no brainer that the drug should always be preferred to the placebo.
A Stanford infectious disease professor told me he thought it was criminal that docs are saying “we will wait for the phase 3 study on fluvoxamine” when the evidence on the table is so compelling.
I agree. It is criminal to wait around for months for completion Phase 3 trial when we have a FDA-approved drug with a 27 year safety record that has been proven in multiple independent trials to be 100% effective in eliminating hospitalization and death from COVID and there are at least 4 observational studies confirming that the drug is effective, and we have no data whatsoever showing the overall risk > benefit (not even a single anecdote).
It's so compelling that top officials at NIH would take the drug if they got COVID. But they are not allowed to say that as public policy. I know med school deans that would say the same privately.
We have no clue what NIH Guidelines group is thinking. I would love to see THEIR calculation as to how they can conclude that they will save more lives by keeping fluvoxamine off the guidelines vs. adding it for use in "shared decision making." Exposing that calculation would be in the public interest... tremendous public interest. But no member of Congress seems to care enough to ask. Asking that one question can save hundreds of thousands of lives because it would expose the hypocrisy: that the CDC is out to protect their reputation and NOT American lives. The math doesn't lie.
Here's a simple analogy. Suppose a gunman walked up to you and pointed his gun right at you and is about to pull the trigger. At the last minute someone hands you a bullet proof shield. They tell you it has been tested 157 times in a row and in each and every case, the bullet was deflected and the person's life was saved. What would you do? Would you hand back the shield and say, "No, I need more evidence"? Or would you take the shield and use it immediately?
This is the question in front of the FDA now. The gunman is real: he's killing 90,000 people a month right now. The evidence on the shield is real. The FDA is now considering whether to grant an EUA.
The FDA must weight both risks and benefits:
- Taking the drug is less dangerous than taking a tylenol (500 people die a year from tylenol overdosing whereas there are no reported deaths from fluvoxamine overdose in the scientific literature).
- The efficacy was 100% in multiple studies (both published in peer-reviewed journals) with min effect size of 75% with 95% confidence.
To most people, the decision couldn't be more obvious that the benefits outweigh the risk. A key opinion leader group that met on Jan 22 (leaders from NIH, CDC, and academia) took just an hour to review the evidence before supporting with more than a 2:1 margin that fluvoxamine should be part of a shared decision-making process between doctor and patient.
The FDA could reason "no need for us to act because each hospital will independently add fluvoxamine to their treatment guidelines." But that's a bad assumption. No hospital will take the reputational risk. Neither will big telemedicine providers. Even if fluvoxamine had 10,000 patients in a row without hospitalization or long term symptoms at a large clinic and even with 20 observational studies with huge effect sizes and low p-values, the data would be ignored. You'd never even get an opportunity to present the existing data for the panel. You'd be told, "come back later when you have phase 3 data. We don't care how many studies you have, the effect sizes, or p-values."
If you're convinced an EUA will save lives, feel free to stop reading here. If you aren't, here are the details.
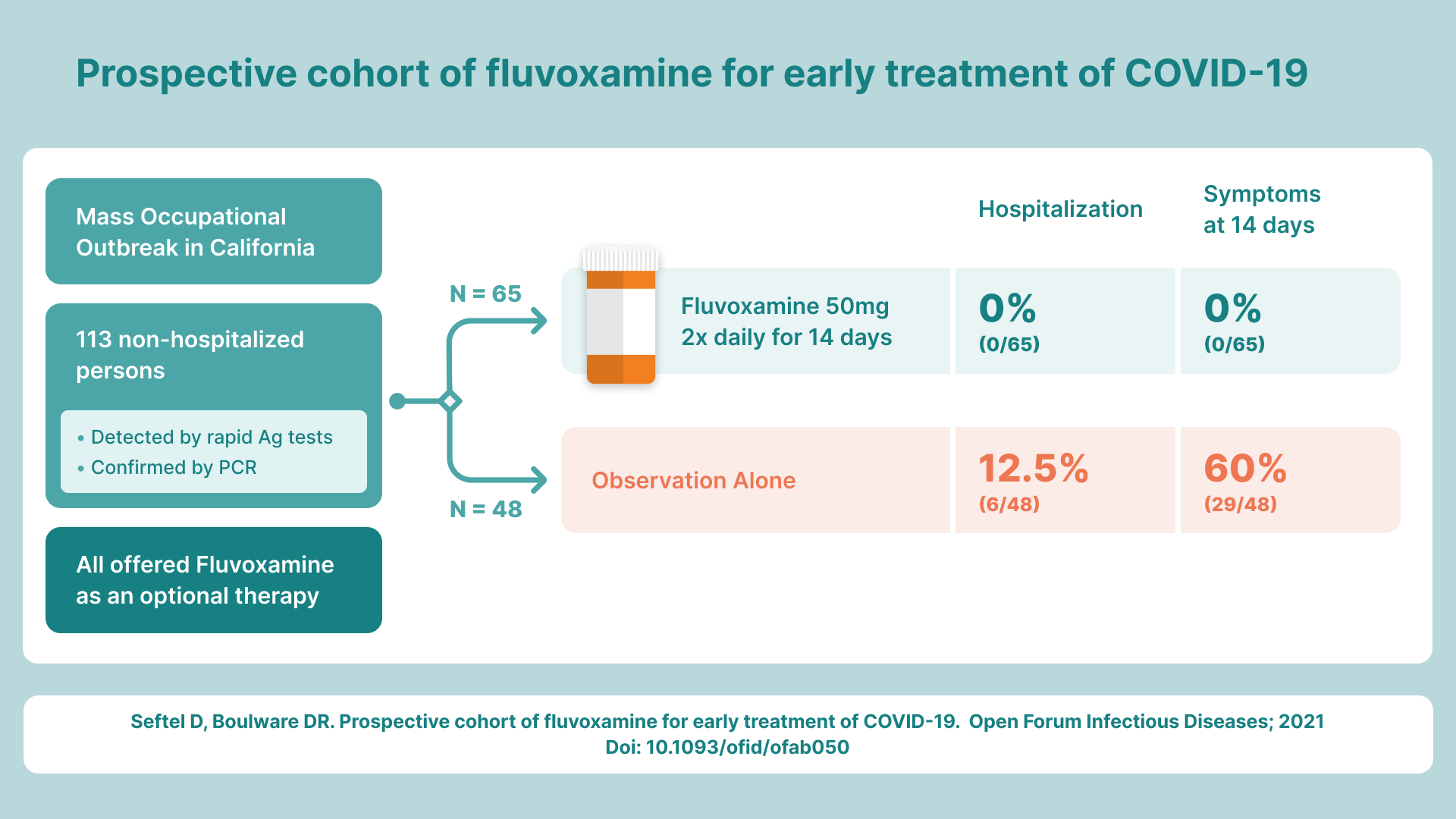
Fluvoxamine (50mg BID for 14 days) was shown in the Seftel real-world evidence trial to be 100% effective in preventing hospitalization and death from COVID vs. 12.5% in the no treatment group. This confirmed an earlier RCT published in JAMA showing a similar 100% effectiveness. No other drug has anywhere close to these numbers in multiple independently run trials. Both trials were published in peer reviewed journals and both were hailed as "Editor's Choice."
It is the inflammation caused by the virus that wreaks havoc on your body, not the virus itself. What fluvoxamine does is basically "activate the fire department" to dump water on the fire (the inflammation).
Fluvoxamine has been shown to be effective in reversing COVID at all stages. As of Feb 1, 2021, I have never heard of any case where it failed at any stage, which includes hospitalized patients on high flow oxygen and intubated patients. It takes longer the sicker you are, but it seems to always work. The sicker you are, the higher the dose that is appropriate, and the longer it takes to work.
Fluvoxamine can be started at any stage, but obviously, the sooner it is started, the lower the risk of any collateral damage. To use a simple analogy: the sooner you put water on the fire, the less collateral damage there is.
We don't know the true effect size of fluvoxamine because the number of patients in the studies is relatively small (N= 157). The true effect size of fluvoxamine is highly likely to be at least a 75%. Therefore, fluvoxamine will likely be a key part of an effective "cocktail" to fight COVID that involve other repurposed drugs that are now being investigated (such as ivermectin, mirtazapine, cyproheptadine, metformin, doxazosin, fenofibrate, disulfiram, camostat, SLV213, GS-441524, and more).
Doctors should discuss fluvoxamine with all COVID-positive patients as early as possible in the disease since it can be prescribed off-label today, is very safe when used as directed, and costs only $10 (without insurance).
There is a lot of evidence supporting how this drug works. For example,
- We knew in February 2019 that fluvoxamine reduced damaging aspects of the inflammatory response during sepsis and protected mice from lethal septic shock (work done by Professor Alban Gaultier at University of Virginia)
- observations made May 2020 in the Sainte Anne psychiatric hospital showed more than a 3X difference in severe COVID infections between the staff and the patients
- A retrospective observational study done in France by Nicolas Hoertel found statistically significant and very large protective effect observed for SSRIs. And the SSRIs protection ability was the exact same order as their Sigma-1 agonist ability. The chance of that happening by pure luck is 1 out of 120. The protection for fluvoxamine is estimated to be around 75%, consistent with the clinical trials.
- A German study (see Figure 1) showed a very large protective effect of having a comorbidity of depression (since it is associated with SSRI use). So all antidepressents as a group lowered risk by 36%, so it's not hard to imagine that the most effective antidepressant is many times that.
- There are many anecdotal examples, some of which are impossible to explain if there wasn't a real effect. For example, 1) every single patient returns to near normal within an average of 3 days after starting the drug 2) the intubated patient who got instantly better when the drug was given and as soon as the drug was removed deteriorated and died within days). While individually each anecdote of them could be dismissed, there are simply too many examples to dismiss. And there are no anecdotes of no effect.
In short, multiple different observational studies show a huge effect, the Lenze RCT shows 100% effect size, and the Seftel RWE study done right after the RCT confirmed the RCT again with a 100% effect size. And much more. It's pretty hard to imagine that there isn't something big going on.
Arguments for waiting for the phase 3 trial outcome
There is a phase 3 trial for fluvoxamine that is taking a long time to enroll (enrolling 10 a day means it takes 100 days to enroll the # of patients required).
So the question is: Should we wait for the Phase 3 trial results or just look at the data on the table today and make a judgement call? That is the key question this article addresses.
The main reason that people wait for a phase 3 is that overall, only 60% of phase 2 drugs are confirmed in Phase 3 trials. That is why a Phase 2 result is treated as speculative. But that statistic is a general statistic and it disregards all the supporting evidence for a specific drug. It assumes the only thing you know is “it passed a phase 2.” When you have a drug with the quantity and quality of evidence that fluvoxamine has demonstrated, the success rate in Phase 3 is much higher. I always ask skeptics "so when have you seen a drug with this much data fail on phase 3?" and they can never point to a relevant example with a similar fact pattern of evidence. The conversation always ends there.
The more relevant objection would be "for drugs with this sort of fact pattern where there are 8 plausible mechanisms of action, multiple observational trials showing very large statistically significant effects, as well as RCT , RWE, and anecdotal evidence that is all 100% positive, and where every patient with symptoms turns around in 3 days, what is the success rate in phase 3?" That's the question. And the answer appears to be "well, that has never happened." This makes it clear that waiting months for a phase 3 is simply a needless waste of human life.
There are very relevant things that don’t make it into the EUA application that are extremely important. For example, for the 77 patients with symptoms, they all resolve within ~3 days after treatment… that’s spectacular showing a very strong effect and consistent effect and that is very highly unlikely to be reversed in a phase 3.
I asked Dr. Seftel, "what's the chance that fluvoxamine will be confirmed in a Phase 3 trial?" To my surprise, he said, "probably over 90%." I asked him why he said that, and he explained that there could be compliance issues with remote clinical trials whereas real-world evidence trials are much more reliable since the doctor has direct contact with each patient.
So I asked a different question, "What do you think the chance is that we just got lucky and there is truly no effect?" His answer was more direct: "Slim to none; there is just no way that these observations can occur by chance."
Who should take the drug? Only those with multiple risk factors?
When you have the flu, everyone should take tamiflu as soon as possible to minimize your downtime and minimize your chance of hospitalization or death. There is not a criteria to "only take tamiflu if you are older, or have more risk factors, or only if you have severe flu symptoms." You basically always take it because it always improves things (at least that is the belief, and it may be wrong but that's another story).
The same is true for fluvoxamine. If you have a fire, even if a small one, put the water on it immediately or you will risk irreparable collateral damage.
Because we can't tell in advance who is going to have a difficult time with the virus, since the treatment with fluvoxamine has mild, temporary side effects at worst, it appears prudent to give it to any patient in which it is not contraindicated (e.g., drug interactions, mental state, etc.).
The case for granting an EUA: benefits>>risks
EUAs are given in emergency situations when the FDA believes that the evidence available demonstrates that the benefits outweigh the risks.
Here are some reasons I think it makes sense for the FDA to issue an EUA.
The most basic reason is that with a very short dose (14 day) and low dose (50mg BID), the known side effects are limited, mild, and temporary. We know this from 37 years of experience with the drug on over 10 million people. The benefit, as demonstrated from the evidence available, is a 75% or more reduction in the risk of hospitalization/death (in both studies, the effect size was 100%, but the Fisher Exact test on the combined data gives a 95% confidence of an effect size of 75% or more).
If you look at the Seftel study and label the cohorts as Drug A and Drug B, and all the side effects are listed, I believe that everyone would choose fluvoxamine.
We saw this in real life in Dr. Seftel's study where at first only 40% of patients opted for the drug and just two weeks later, 100% opted in, with many proactively asking for the drug. It was obvious to the patients that the benefits were huge and the risks very minimal; no in-depth research was required: they could see the difference with their own eyes.
Dr. Seftel observed that every single patient in the treatment group recovered within a few days, their brain fog cleared up almost immediately, and the most common complaint was that the doctor was being unreasonable by not allowing them to return to work.
There are some things you can't really communicate in the numbers that end up in papers. Dr. Seftel called the result "truly the most extraordinary therapeutic effect I’ve seen in over 25 years of practicing medicine." How can you quantify that? Sure it's a subjective statement, but how often do you hear that in medicine? Must we only consider evidence that we can objectively quantify?
Furthermore, there are rational explanations for the mechanisms of action of this drug. Eight different mechanisms are hypothesized but the main ones being the reduction of circulating serotonin and the activation of the sigma-1 receptor. This explains why as a class, SSRIs are protective, with the greatest protection being those SSRIs with the most Sigma-1 activation. This is clearly shown in the Hoertel paper.
Here's an explanation of the Sigma-1 activation effect:
The endoplasmic reticulum is somewhat like a factory for building proteins into their final form. In general, chaperone proteins can assist in proper folding and maturation of other proteins that are being produced in the endoplasmic reticulum. One S1R function is to act as a chaperone to facilitate the maturation of certain proteins. But it also has other functions, such as regulating the actions of other ER proteins (including a protein called IREI that that is involved with the ER stress response and inflammatory responses). IRE1 is a protein that can activate another protein called XBP1 which can then promote the production of cytokines. In the presence of a S1R agonist, S1R can essentially turn off IRE1, so IRE1 will not activate XBP1, so that the cytokine production will decrease.
An expert panel of people from NIH, CDC, and academic institutions convened by Dr. Jeffrey Klausner reviewed the evidence for fluvoxamine and a post-meeting survey showed that the infectious disease experts supported its use by more than a 2:1 margin. This is another data point supporting the contention that the benefits outweigh the risks.
Why do we need an EUA? Docs can just give off-label today
Yes, that's right, docs can prescribe fluvoxamine off-label today no problem. But only 280 people read the Seftel study that confirmed the Lenze RCT. So it won't happen today. The press still won't cover it because it is deemed experimental. An EUA will totally change the dynamic.
Let's us review the key characteristics of doctors (Dr. Klausner provided items 1-5 and note this list doesn't apply to surgeons):
- Docs are sheep
- Docs are risk averse
- Docs don’t like to take chances
- Docs don’t like to be experimental
- Docs don’t like to thought of as cowboys
- Docs are afraid of legal consequences of doing anything not CDC approved
- Docs are unable to change their behavior when circumstances change
The EUA gives doctors plausible deniability. It means that docs don't have to spend hours researching the drug because the FDA already has done the research and made a determination. An EUA changes everything; it means that more docs will be willing to prescribe the drug, more patients will ask for the drug, the media will now be "allowed" to talk about the drug, YouTube videos that mention the drug will no longer be taken down, etc.
And that means more lives will be saved.
Dosing
Nobody knows the optimum dosing of FLV for COVID. The 50mg BID x 14 days dose used by Dr. Seftel was very effective with no failures. Everyone turned around back to near normal in a few days. So there is currently no need to increase the dose since you can't get any better than 100% response. All increasing the dose will do is introduce side effects that will lead to patients not being compliant.
It's possible that the optimum dose for COVID is even lower than 50 mg BID. I would not be surprised if the optimum dose were only 50mg QD.
All too often, we make the mistake in clinical trials that more must be better. But this is almost never true. There are always limits. Too much water can kill you, too much insulin will kill you, too much food will kill you, too much of a chemo drug will kill you, too much of a sleeping pill will kill you, etc. I found that when I cut my cancer drug (Ibrutinib) dose in half, the drug actually was more effective. The goal of dosing is to give just enough to solve the problem and no more since you want to avoid collateral damage and unintended consequences.
By recommending a fairly modest dosing of 50mg BID, we can go up or down from there based on patient responses. When properly dosed, the patient should be able to tell an improvement from day to day.
Fear of doing something new
Many doctors are fearful of any drug that they have never prescribed before because they read a long list of side effects that are possible. But those side effects are based on long-term usage of the drug at the maximum approved dose, rather than acute usage (14 days maximum) at a significantly reduced dose (1/3 the approved dose).
The doctors who are the most familiar with fluvoxamine are psychiatrists who maintain that the side effect profile is quite minimal (mild nausea in a small percentage of case) and resolve quickly once people stop taking the drug.
So we have a case of very minimal risk and a potential reduction of 75% or more in the hospitalization and death rate. That makes for a compelling case especially against the risks of the "no treatment" option (significant risk of hospitalization and death).
Fluvoxamine isn't a perfect drug. You don't want to drink coffee while on the drug or you will be really wired (increases caffeine half-life from 5 hours to 31-36 hours so no more than one very small cup in the morning; abstaining is even better). People metabolize the drug at different rates and different people have genetic differences in the serotonin transporter or sensitivity to serotonin. But the 50mg BID dose seems like a very good compromise "one size fits all" prescription that even works for children (this is 1/2 of the max dose for children) and in Seftel's study provided 100% protection from hospitalization in all patients.
When prescribed in accordance with the known prescribing guidelines, there is no known risk of death. Side effects of SSRIs typically resolve after the dug is stopped, and if a person is only taking the drug short-term and at 1/3 of the authorized dose, there is even less chance of any lingering side effects. By contrast, the risk of death (and permanent side effects) for every person who has an active COVID infection and has not been vaccinated is always non-zero.
But since most doctors have never prescribed fluvoxamine, they will go to a page, read about all the side effects and conclude it is a dangerous drug.
I asked Eric Lenze about the safety level of SSRIs (and he's responding for much higher dosages than we are talking about here):
I've prescribed SSRIs thousands of times somewhere in the 5-10K range. Rarely people can get hyponatremia (low salt level in the blood), or serotonin syndrome (too much serotonin signaling in the brain). SSRIs also impair platelet function.
A permanent side effect would be a secondary indirect effect -- for example, if you got a hemorrhagic stroke because the SSRI impaired platelet function.
A good way of thinking about SSRI risk is like if you took an aspirin a day for 2 weeks. It seems benign (because it is), but if you happened to get in a car accident or have a bad fall in those 2 weeks, you'd be more prone to bleed. (On the other hand, you'd be less likely to have a heart attack or ischemic stroke for that same reason).
If SSRIs help vs. COVID, their benefits far outweigh any risks. No medication is 100% safe, not even Tylenol, but these are pretty close.
So when we consider these very rare and very minor side effects (for an acute dosing at 1/3 of the authorized dose for OCD), you should also take into account the benefits of the SSRI even if there was no COVID benefit whatsoever because it reduces the chance of a blood clot that could kill you. The bottom line: it's about as dangerous as taking Tylenol for the same number of days.
Also, any mental impacts take at least 3 weeks after being on the drug at a high dose. So again, this further bolsters the safety assessments.
Even if a person has been vaccinated and gets COVID (and thus has virtually no chance of dying from COVID), it seems pretty clear from the data on the table as to the proven ability to eliminate mental fog and prevent and inflammatory response (without any significant short or long term effect) that the person would be better off taking fluvoxamine that avoiding it.
In my opinion, anyone who can qualify to take this drug (no drug interactions, mental state, etc) should take it as soon as possible after they have a positive COVID test result because it is always better to treat a virus early and mitigate damage early than to sustain damage and deal with the potentially permanent side effects including brain damage. Furthermore, a third of recovered patients from COVID will return to the hospital within 5 months and 1 in 8 die.
On the day scientists can predict with 100% certainty, in advance, which patients will or will not suffer from brain damage, which patients will be hospitalized, and which ones will die, then fluvoxamine could then be limited to those patients at risk.
But until that day arrives, in my opinion, it is far better to err on the side of caution by giving the drug to all qualifying patients (and knowingly have a very small risk mild nausea for a few days), than to risk even a small chance of death. After all, if it is safe for those who are at the highest risk, it should also be safe for those who are more healthy with fewer comorbidities because those people can die too. If we ever get to a point where we have evidence that for a certain cohort the death rate on drug exceeds the death rate off-drug, then it should be restricted.
Therefore, my contention is that the case for fluvoxamine is both clear and compelling and there is no reason to wait. If later it turns out that this analysis is wrong, the FDA could immediately revoke the EUA.
If fluvoxamine is very effective, can we unmask?
We don't mask up for influenza, and influenza kills 30,000 people each year. Therefore, if fluvoxamine (or some combo), is shown to be more than 95% effective or after most all of us are vaccinated, then it would be reasonable to think we can get back to "normal."
Conclusion
For now, it is a certainty that doing nothing will lead to 90,000 deaths over the next three weeks. Giving people 50mg BID fluvoxamine for 14 days could likely save more than 67,000 lives over the next 3 weeks at the expense of some people will have mild nausea while on the drug.
Death is not a reversible outcome. The math (Fisher exact test on the combined dataset) says there is more than a 95% chance we can save most of those lives by issuing an EUA. We should follow the math.
If someone can show data however, that the use of fluvoxamine increases the death rate, that would be a reason not to issue an EUA. But no such data exists as far as I know.
The FDA should rule on the data on the table today. The choice is obvious and there is little reason to delay.
Given a choice between fluvoxamine or no treatment (the current standard of care), the evidence we have in front of us on the table today, is crystal clear.
Every week we delay this decision another 30,000 lives are lost. The FDA should consider that fact in deciding how much time it needs to take to make an obvious decision.
Dr. Jeffrey Klausner is a very highly respected Professor of infectious diseases. He was quite skeptical when I first told him about fluvoxamine. The more he read about it, the more convinced he became. He convened a key opinion leader meeting. The outcome of that meeting led him to write an op-ed published in the Washington Post calling for the off-label use of fluvoxamine in a "shared decision making" process between doctor and patient.
This is the right call to make and by granting an EUA, the FDA will, in effect, give implicit permission to other doctors to do exactly as Dr. Klausner is doing today. Of course, no doctor "needs" an EUA to follow what Dr. Seftel and Dr. Klausner have done, but the EUA makes it much more likely for doctors to do this. Without the EUA, each doctor will have to do hours of research to convince themselves the drug is effective with minimal risks. With the EUA, the extensive research is less necessary.
There is no doubt in my mind that granting an EUA will result in a huge saving of lives that didn't need to be lost.
But if I'm wrong and there is no effect, then at least we failed fast and can move on to the next most promising drug on the table. Because the drug has a known safety profile, and because the EUA could be lifted rapidly if there is truly no effect, there is little downside in giving this a chance. This is exactly what we should be doing: trying the most promising options on the table rather than waiting for absolute certainty before we act.
If a gunman walks up to me and is about to pull the trigger, and someone hands me a bulletproof vest that they said they tried in the lab 5 times and it worked every single time and urges me to put it on, I wouldn't say, "no, I'll wait for more data."
I sincerely hope the FDA makes a determination quickly. Unless there is compelling evidence that fluvoxamine increases the death rate, we should give this drug an opportunity to succeed. Now.