How I would treat COVID-19
Please see my answer on Quora for the most up-to-date version of this article.
Better still see the article on the home page about COVID treatments.

Please see my answer on Quora for the most up-to-date version of this article.
Better still see the article on the home page about COVID treatments.
Links to important documents relating to vaccine safety and efficacy issues. Highly recommended.

Treating COVID, long-haul, and vaccine side-effects ASAP is key for best outcomes.

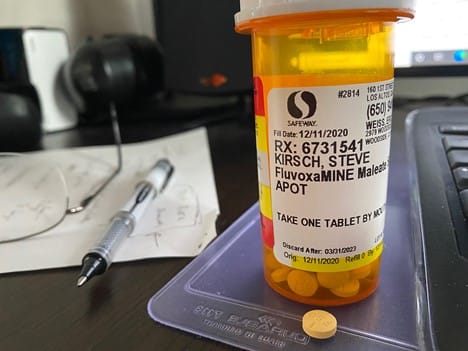
Everyone says "we need more data" to show fluvoxamine works for COVID. Is that really true?

I'm very bullish on two drug combos since it is rare for a single drug to be 100% successful. For example all of these combos should have near 100% success against hospitalization, death, and long-haul COVID symptoms: 1. Proxalutamide and fluvoxamine 2. Proxalutamide and ivermectin 3. Fluvoxamine and