The fast, easy, safe, simple, low cost treatment for COVID that has worked 100% of the time to prevent hospitalization that nobody wants to talk about

We now have a viable solution to reduce COVID hospitalization and mortality; a small pill that transforms this destructive virus into a mild-mannered common cold. But the mainstream media is ignoring the story, including the publication of the study in the top peer reviewed journal in the world (JAMA) that showed it was 100% effective in keeping people out of the hospital. Doctors dismissed the study as just one data point and demanded further confirmation before they would accept the result. Their request was answered just one week later where there was a massive COVID outbreak at Golden Gate Fields in Berkeley, CA. Dr. David Seftel, the Harvard/UCSF-trained track physician for the past 7 years, had read about the study in JAMA, found the study results impossible to ignore, and since the drug is an FDA approved drug that has been taken by over 10 million people over the past 37 years, he offered the drug to COVID-infected employees. Although everyone at the racetrack adores Dr. Seftel, despite his best efforts, only 35% of the patients chose to take the drug. Just two weeks later, the acceptance rate is 100%. Even more impressive is that patients who initially refused the drug are now coming back asking for the drug because they saw the results with their own eyes: this is a remarkably tight-knit community and the difference between their friends who took the drug vs. rejected the drug was as clear as night vs. day. Word spread fast. Anyone who took the drug shrugged off the virus like a mild cold. The local Berkeley newspaper observed that the hospitalization rate was so low (“a handful of people”) despite the massive number of COVID-postive cases. This was what I like to call the “Miracle at the Racetrack.” The newspaper wasn’t allowed to contact the employees to find out why. So nobody knew what caused this miracle. When Dr. Seftel attempted to inform physicians of the secret behind this success, he was ignored. Many doctors are trained to totally ignore any evidence that is not in a clinical trial. Even if everyone died who didn’t take the drug, they would still not consider the drug to be effective (I’m not kidding; it’s really that bad). I believe that it is bad practice in a pandemic to ignore evidence just because it wasn’t performed in the context of a randomized clinical trial. We should not be giving a zero weight to a pseudo-randomized trial where the randomization favors the placebo. We are allowing hundreds of thousands people to be hospitalized and die unnecessarily. And the press refuses to cover this story because they are focused on the vaccine and don’t want to raise false hopes of a cure. Everyone waits for publication in peer reviewed journals. That takes months to happen. It's stronger evidence once published, but we should be considering all the evidence and the mechanisms of action. They think they are protecting the public, but they are not. They could be reporting what happened and noting "the paper has not yet been published but it comports with multiple observational studies from multiple countries." Please spread the word because the mainstream media has failed us.
In March 2020, when COVID hit, I sought to find a cure for COVID by starting the COVID-19 Early Treatment Fund (CETF). With the help of a world-class scientific advisory board, we funded 14 projects that were selected from over 60 grant proposals from top scientists from all over the world. We got lucky and succeeded beyond our wildest dreams! One of the research teams we funded, Dr. Eric Lenze and Dr. Angela Reiersen, ran a randomized control trial (RCT) and discovered that fluvoxamine, an FDA-approved pill that has been around for 26 years, turns COVID into nothing worse than a common cold.
It worked consistently. In two clinical trials involving over 260 people: Zero hospitalizations, Zero deaths in the treatment group.
So far, the drug has worked 100% in multiple studies, was published in JAMA (selected from over 10,000 submissions on COVID), it’s obvious to any lay person who is allowed to observe both the treatment and control groups that it has worked spectacularly well, and there is solid science behind it. It has been replicated with near identical stats, it can be prescribed today by any doctor. It costs only $13, before insurance. Available now.
I thought it would be the biggest story of the century. Not only was it a big story, but the timing couldn’t have been more perfect: it came JUST IN TIME TO AVOID THE BIGGEST HEALTHCARE MELTDOWN in US HISTORY. To my utter amazement (and to the amazement of my friends in the media including a 9-time Emmy Award winner who has tried in vain to get any of her friends to even look at the story), the mainstream media ignored it so nobody knows what it is. An effective treatment for COVID is hiding in plain sight, but nobody wants to hear it.
For example, one news organization said the fluvoxamine story was “interesting, but we are 100% laser focused on the vaccine.” Yet despite their “laser focus on the vaccine,” they air gut wrenching human interest stories of family members recalling people who have died. Yet they are not willing to air a much more uplifting story — of the lives saved at the Golden Gate Fields racetrack thanks to the efforts of a heroic and extraordinary doctor who had read the JAMA study the week before and realized this new data was clear and compelling that this drug was far better than giving his patients nothing and seeing them end up in the hospital or in the morgue.
People assume that any legit treatment would be top news that everyone would know about. So if you claim to have a treatment that has worked 100%, then it is assumed you must be spreading fake news. One of my friends, Mark Travis, shared my article with just 100 friends and it was removed 30 minutes later.
A few days later, Medium revoked my account, removed everything I've ever published over the past 6 years, and told me never to come back. Many doctors sent notes to them saying the information was valuable and accurate. Patients sent notes saying they needed that information to help people with COVID. That made no difference.
Dr. Joshua Vogelstein, Assistant Professor of Medicine at John Hopkins, told me not to despair that people aren't paying attention. He said even if the press ignores this drug for COVID use, the drug we discovered has potentially game-changing applications in many other diseases involving an inflammatory response that kill (or disable) far more people each year than COVID including:
- Influenza (40K deaths/yr)
- Future pandemics
- Sepsis (11M deaths/yr, far more than COVID)
- Pneumonia (3M deaths/yr worldwide)
- Heart attacks (859K deaths/yr)
- Alzheimer’s disease (122K deaths/yr)
This drug may well be the story of the century, yet nobody will touch it today. The RCT study published in JAMA has not been attacked; nobody has identified any confounders that could negate the result. And it has worked, so far, 100% in practice.
That in a nutshell is the COVID story that I have to tell.
The reason the press doesn’t write about it is simple: many mainstream doctors discount anything but RCT evidence. For example, when Dr. Seftel asked if he could share his lifesaving story with other physicians on a popular YouTube COVID podcast aired by a top California university, he was told to come back when there was a phase 3 clinical trial involving hundreds of patients.
That's insane. On Jan 17, the CDC said we'll lose 90,000 American lives over the next 3 weeks. Even if this drug worked just 50% of the time, that's 45,000 lives that could be saved. But we are not even given a forum to educate the doctors on the confirmatory study.
Dr. Joseph Ladapo wrote eloquently in the Wall St Journal about what a mistake this is. If there isn't phase 3 trial data, the press doesn’t want to promote junk science or false cures because it would tarnish their reputation and/or raise false hope. They believe that it would be a disservice to their listeners. In normal times, they might be justified in having that attitude. But this is a pandemic where people get hospitalized, our hospitals are overrun, and people are dying. So instead of just dismissing a credible claim, maybe it would be prudent to make a phone call to that racetrack doctor and check it out? Just three people from the press did that. As of Jan 17, no story has been allowed to be published even though the study had results on December 1.
The press need go no farther than the racetrack at Golden Gate Fields to ascertain the truth. This drug has worked so well that it is obvious; 100% of the employees chose the drug (up from just under 40% 2 weeks earlier). How often do you see that kind of a change in beliefs? It would be like everyone, in just two weeks, agreeing on the same Presidential candidate. But it’s even more powerful than that. Even people who refused the drug when it was first offered, changed their mind and opted for treatment. Because people talk. And they can see with their own eyes what is going on. It is obvious and dramatic. Yet to a trained doctor, nothing whatsoever happened of significance. It is a non-event because it isn’t in a randomized control trial. 100% of that evidence must be ignored and discarded.
The press doesn’t have to worry about its reputation. It can simply report that facts: that, so far, this drug has saved people from the hospital 100% of the time it has been used as directed, even when given late in the disease. This is not raising a false hope. It is reporting exactly what happened.
Had this been a clinical trial evaluating two different drugs, Drug A and Drug B, that the data safety management board (DSMB) would have immediately halted the trial because Drug B was clearly putting people at risk and causing them to die. Drug B of course is the placebo. Drug A is Fluvoxamine.
If everyone talked to their doctor about taking fluvoxamine after being infected by COVID, all of data we have now very clearly shows that it would dramatically reduce the hospitalization rate, likely by a factor of 4 or more.
Most doctors will not have time to review the evidence. They will stick to the tried and true and not prescribe anything until it is proven in a phase 3 trial or there is an EUA (because then they can "blame" the FDA).
RCT of fluvoxamine (FLV) done at Washington University (n=152) published in JAMA on November 12, 2020 found that patients taking the drug had a 0% hospitalization rate (vs. 8.3% for placebo). 100% protection from the virus.
Just one week later, another study (n=146) done by highly regarded track physician Dr. David Seftel at Golden Gate Fields of 113 COVID cases confirmed the same 100% success rate demonstrated in the RCT trial. The hospitalization rate for the 53 who chose not to take the drug was over 10%; for the 60 who opted to take the drug, the hospitalization rate was 0% (Not yet published.) Again, 100% protection. Dr. Seftel, called the result “extraordinary outcome” and a “teachable moment” (i.e., that we are making a huge mistake by not acting on the best evidence, albeit imperfect, available at the time that a critical medical decision must be made).
A large observational analysis (n=7345) of hospitalized French patients found that those on SSRIs (of which fluvoxamine is one) had a very substantially reduced risk of death from COVID. (n=257, HR = 0.56.). SSRIs with highest Sigma1 activation showed the greatest protection.
Fluvoxamine is a potent sigma 1 agonist. This paper in Science found that patients receiving another sigma 1 agonist (indomethacin) had a materially reduced likelihood of requiring hospitalization compared to those receiving celecoxib, which doesn’t activate sigma 1. Fluvoxamine is very potent Sigma1 agonist.
Lastly, an RNA screen (not yet published) has found that genes unregulated by fluvoxamine significantly inhibit COVID.
The modest anticoagulant effects of fluvoxamine (via impairment of platelet aggregation, depletion of platelet serotonin levels, and reduction in platelet count) may be responsible for its positive value in counteracting the procoagulant effects associated with significant morbidity and mortality on COVID-19.
Given the combination of clinical, real-world, observational, and theoretical data is all consistent, it’s surprising to me that none of the mainstream media is making this information known to the public. AFAIK, this $13 drug is the fastest, cheapest, and safest way to dramatically reduce the load on our hospitals worldwide and avert a crisis.
The press (Berkeleyside) reported on the astonishingly low hospitalization rate at Golden Gate Fields, but never revealed that the “secret sauce” was the use of fluvoxamine.
The drug’s effect was so profound that the acceptance rate for the drug among the staff jumped from under 40% to 100% in just 2 weeks. People could see the difference in their co-workers. People who formerly refused the drug, are now requesting it.
Fluvoxamine is a very old drug with a long safety record. It was approved by the FDA 26 years ago. There are 12 manufacturers worldwide.
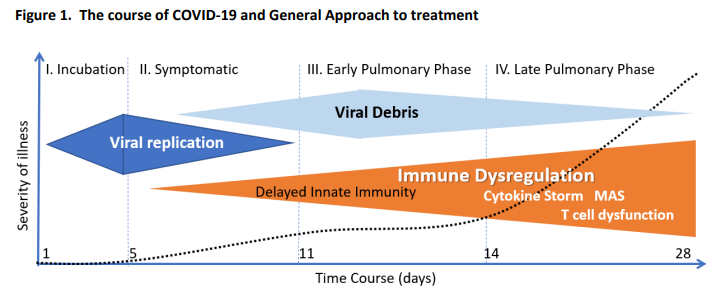
Patients at GG Fields were told to take 50mg of FLV twice per day for 14 days. It’s that simple. That dose (which is much lower than the dose in the RCT, but proven to be just as effective when used at Golden Gate Fields and can be increased if your SpO2 drops) will almost certainly keep you out of the hospital and it will also improve your cognitive abilities. If you are hospitalized (because you ignored the advice), talk to your doctor about the drug. The drug has been successful even on intubated patients that are near death. In general, it is better to start the drug before the inflammatory response starts (the inflammatory response starts 7 days after first symptoms, on average see chart).
If your doctor refuses to prescribe fluvoxamine, you should first point her to this article. If she’s a really good doctor, he’ll read the evidence here, and then watch the 15-minute segment of the video (starting 1 hr into the video), where he can hear directly from Dr. Seftel.
If that doesn’t work, then you have two more options.
OPTION 1: Enroll in the fluvoxamine phase 3 clinical trial (highly recommended since it helps you and others); this is a fully remote trial so you can enroll from home. Top universities like Washington University, Northwestern, McGill, etc. are part of the trial.
OPTION 2: Try another doctor who is more open minded and willing to consider the evidence on the table. This may take a few tries, but they shouldn’t be too hard to find. There are significant drug interactions which your doctor will tell you about (they use UpToDate to find this). I know many top doctors that would take it if they got sick, but will only offer it if the patient ask (they don’t want to “push” any treatment with less than perfect evidence on patients, even if could save their lives. See Got COVID? This drug can save your life but you have to ask for it).
OPTION 3: Use telemedicine at cityhealthuc.com to get a prescription.
Doctors are suffering and dying disproportionately. Your doctor probably doesn’t know about fluvoxamine. By asking your doctor about fluvoxamine, you may not just save your own life, but your doctor’s life as well.
How can the mainstream press justify ignoring this? No outpatient drug has better efficacy data to date. No drug has more consistent data to date. The evidence is compelling and consistent. No confounders have been found.
The moral of this story is that the medical establishment has largely won (so far), even though they are wrong. Dr. Ladapo was absolutely right on the money with his Wall St. Journal op-ed that Too Much Caution is Killing Patients. To completely disregard less than perfect information (so that the real-life study at Golden Gate Fields could be considered as confirmatory to the RCT) in a pandemic is a mistake that hundreds of thousands of people will pay the ultimate price for. And the mainstream media is helping them do that by keeping this information from public view. It's tragic.
Steve Kirsch is an entrepreneur and medical philanthropist based in Silicon Valley. He started the Covid-19 Early Treatment Fund (www.treatearly.org) in April. The fund was one of the sponsors of the fluvoxamine outpatient clinical trial. He has no financial interest in the success of this generic drug.
Related stories
COVID-19 Early Treatment Fund (CETF) Introduction — YouTube
CETF 3 minute intro.
CETF website — Donate
If you’d like to help us continue our important work at CETF, please consider making a donation.