Fluvoxamine: The evidence
Links to evidence about fluvoxamine including the public data repository

It should be crystal clear to everyone that the current CDC guidelines for treating COVID aren't working.
As of January 18, 2021, the CDC estimates that 90,000 Americans will die from COVID in just the next 3 weeks. What's even worse is that a third of recovered patients from COVID will return to the hospital within 5 months and 1 in 8 die.
We need to keep people out of the hospital in the first place. The only way to do that is to treat them as early as possible with a drug that prevents hospitalization and death.
To date, the #1 drug with the most evidence to make a significant difference, without any doubt, is fluvoxamine. It has shown to be 100% protective of hospitalization in 2 clinical trials. In the second trial, it was shown to be 100% effective in long-haul COVID symptoms: None of the treated patients had any long-haul symptoms after 2 weeks compared to 60% of untreated patients having 1 or more of the 15 long-haul symptoms after two weeks, and 29% having 4 of more of the long haul symptoms after 2 weeks.
While these are stunning results, less than a dozen doctors in the US are prescribing fluvoxamine today. All have had a 100% success record in keeping their patients out of the hospital. The other doctors aren't using it either because they don't know about it or fear doing anything not approved by the CDC for treating COVID.
I've collected fluvoxamine evidence here for convenient access. There is an executive summary below, but the most important thing is that top infectious disease docs who have looked at all the evidence (including the two clinical trial results) believe the effect size is 75% or more in reducing the hospitalization rate. This should be top news, but the press is ignoring this and attempt to write stories about it are killed by the editors. See more below.
Reference materials
Fluvoxamine public data repository: The fluvoxamine public repository has all the documents related to fluvoxamine for COVID, including the RCT, RWE, observational studies and a link to the 1 hour lecture on serotonin and fluvoxamine.
Fluvoxamine review


Paper reviewing the evidence and mechanisms of action for fluvoxamine:

Lenze Phase 2 RCT published Nov 12, 2020:

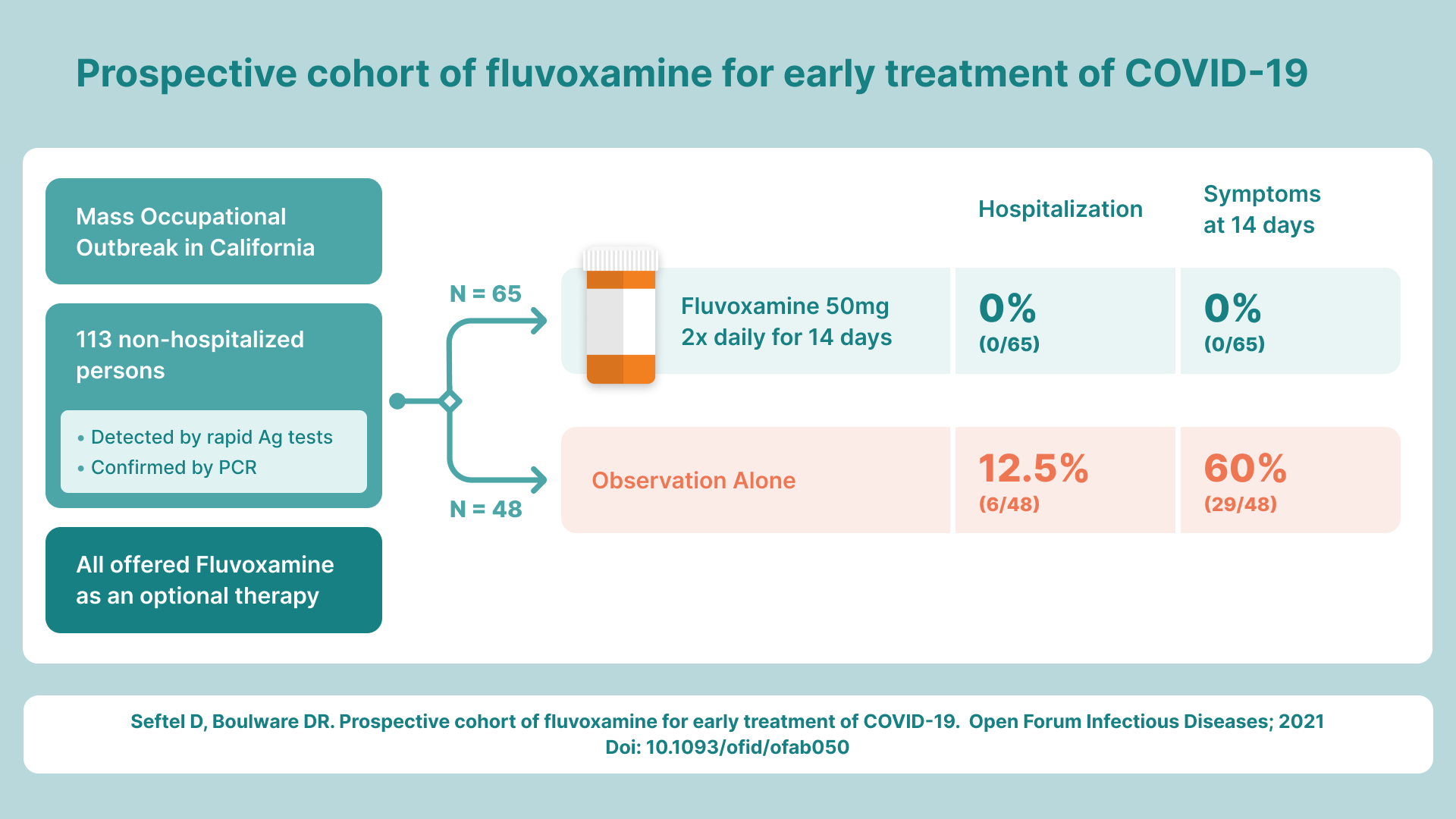
Seftel RWE trial that confirmed the Phase 2 trial published Feb 1, 2021. Note that a total of 77 people got the drug, not 65. There were IRB rules that required the 65 patients to be listed in the diagrams and charts.

Summary of key evidence. Summarizes the 5 observational studies, RCT, RWE, and some of the more interesting anecdotal data
This is a more comprehensive look at the key evidence supporting fluvoxamine:
Hear from the doctors who did the studies directly as well as the Dean of Medicine at Emory University:

List of the best evidence-based COVID treatment options. This is the #1 ranked best answer to "COVID treatment" on Quora:
Presentation on how fear of trying something new is what keeps us shutdown and leads to unnecessary loss of life:

Executive Summary
The Lenze fluvoxamine RCT that was published in JAMA on November 12, 2020 showed a 100% success rate in preventing hospitalization. But an Editor's Note urged physicians to treat this as a hypothesis and not as a basis for clinical decision-making. That was a big mistake because the original paper contained text related to earlier studies and the editors chopped it out. So instead of this paper being treated as confirming an earlier hypothesis, it was treated as generating a novel hypothesis.
Less than a week later, David Seftel read about the Lenze trial, and ignored the JAMA advice. He applied the drug to a large COVID outbreak at Golden Gate Fields just days after the Lenze trial was published. Seftel was able to duplicate the 100% protection from hospitalization and death in the treatment group, vs. a 12.5% hospitalization/death rate for the No treatment group. Seftel used a 50mg BID dosing for 14 days which was one third of the max dose used in the Lenze study. At that dose, no side effects were reported for his patients (I know of only one person who had mild nausea at that dose) and everyone reversed out their symptoms in an average of 3 days.
Dr. Seftel's paper has been accepted for publication and will appear in OFID in early February. Here is the latest version.
This is quite stunning because the PK of the drug done at the Gates Foundation shows it only reaches 50% of the final concentration after 3 days. This suggests that a 50mg BID loading dose for day one, followed by 50mg QD dose for the following 13 days should also be quite effective. This 1/6 of the dose the FDA has approved for OCD (the labelled indication for fluvoxamine)!
Dr. Seftel is an NIH-funded researcher and an NIH reviewer. He said of his study, “This is the most extraordinary effect I’ve seen in my 25 years practicing medicine.”
There are now 5 independent observational studies that show that the drug works (2 in France, 1 in Germany, 2 in the US). See the repository above.
There are at least eight mechanisms of action that we think contribute to the effectiveness of this drug. The documents in the data room discuss all eight (you'll need access to the restricted area to see the presentation on all 8). From the French observational data (see the very last page), it appears that the biggest effect is limiting serotonin release (any SSRI will do that). This alone will give roughly a 50% effect size and explains why all of the the SSRIs are effective including those that do not activate the Sigma1 receptor (e.g., Paroxetine). The next major effect is that that fluvoxamine activates the sigma-1 receptor. S1R can essentially turn off IRE1, so IRE1 will not activate XBP1, so that the cytokine production will decrease. This give another 50% of benefit.
As noted before, the repository has a link to the 1 hour serotonin lecture. It's hard to ignore this lecture in explaining why the drug is so effective.
In the studies and the anecdotes I am aware of, everyone reversed symptoms within days of getting the drug.
Although the average effect size is 100% with a p-value of <.0001, The Fisher exact test on the combined data suggests that there is a 95% chance that the effect size is at least a 75% reduction in hospitalization rate.
I only know of a few doctors who prescribe this off-label, all with 100% success rates. Doctors are afraid that even with a 37-year safety record of this drug, that something will go terribly wrong and they will be blamed. Also, this drug is only prescribed by psychiatrists so most doctors have no experience whatsoever with the drug.
The repository goes over the prescribing guidelines, contraindications, and describes the effect on caffeine consumption while on drug (basically you want to avoid caffeine while on the drug).
There may be a depression of libido while on drug, but since the drug is taken on acute basis, this is only temporary and it reverses once the drug is stopped.
90,000 people don't have to die in the next 3 weeks. But fear of trying something new prevents any doctor from giving this drug a try. People are dying because of physician fear of a new treatment with a 100% success rate and a solid mechanism of action.
The data we have today with just 2 clinical trials (RCT and confirmatory RWE) is compelling. The 5 observational studies is icing on the cake. The anecdotal data of 100% success rates is further icing on the cake.
To date, we have heard nothing suggesting the drug doesn't work or could be harmful.
We should not wait for the Phase 3 RCT. It has enrolled only 130 people in the first month and is enrolling only 70 per week now. It will be months before enrollments are complete. The data is there in plain sight for anyone to see today.
90,000 people will die in the next 3 weeks alone if we continue to ignore this drug that has caused no harm. It is perhaps the greatest unnecessary loss of life in American history.