Got COVID? This drug can save your life… but you have to ask for it!

Say you just got diagnosed with COVID. Your best bet is to run to your doctor and see if you can get a monoclonal antibody like bamlanivimab or casirivimab +imdevimab. But chances are pretty good you won’t be able to get either due to supply reasons (enough for <1% of diagnosed patients) and criteria reasons. So you ask your doctor, “OK, what is my next best option to avoid hospitalization? I’m open to anything. What would you take if you got COVID?”
If you have a top doctor, she will objectively present the evidence by saying that there was a new double-blind randomized clinical trial (RCT, the “gold standard of evidence”), just published in a respected medical journal (JAMA), that tested two existing FDA-approved drugs against COVID. She tells you that one of these drugs is at least as good as, if not better, than doing nothing. She warns you that the trial was small (152 patient), but subsequently, the results have been duplicated in the field with the same 100% success rate. If you combine the results, there is a 99.92% statistical confidence that the benefits are legit (and not due to random chance). She shows you a chart of the results of the RCT:
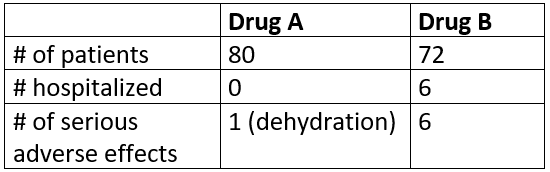
She then asks, would you like to try one of these? Most people would say, “Are you kidding me??? Of course, I’ll choose Drug A! That’s a no-brainer. It’s clearly superior in every metric than Drug B and nobody had to be hospitalized! Who in their right mind would ever choose Drug B?”
Your physician then reveals to you drug A is fluvoxamine, and drug B is a placebo. Now that you know that, did you change your mind? I doubt it!
A trained physician would similarly come to the same conclusion. However, once it is revealed that Drug B is a placebo, then the results would be deemed indeterminate and not suitable for discussion with patients until a larger study is done!
Why you have to ask for fluvoxamine by name
Unfortunately, this scenario would only happen today if you specifically asked your doctor about fluvoxamine by name. There are four reasons for that:
- Even though the results were stunning (“as good as it ever gets”) with a jaw-dropping reduction in the hospitalization rate of 100%, physicians discount the result because it was a small study (N=152). That is still true even after the study results were replicated by other doctors in the field! In short, even when nobody has been able to articulate any confounders that would change the outcome and every experiment was overwhelmingly positive, your doctor will ignore the evidence. This is how they are trained: for non-pandemic times.
- No doctor has the time to do the research to connect all the dots with the abundance of other independent evidence that is confirmatory. If they did that, they see the study as “over the top” evidence. A top biostatistician at John Hopkins did exactly that and concluded that the evidence was compelling enough that she would take it if she got COVID. But since nearly all doctors have no time to connect the dots and because the published RCT study itself did not include that research, doctors interpret the study as a single isolated data point. See [1] below for the details on all the evidence.
- Hydroxychloroquine has poisoned the well and reset the bar. The bar for using new evidence for treatment was initially lowered because we are in a pandemic with no good solutions. But HCQ has reset the bar back to what is was pre-pandemic. So even if you have a study with over 99% confidence of a benefit, that study will be ignored and it is considered bad practice to broach it with patients.
- Doctors have difficulty telling good data from bad data. For example, I was shocked reading the 23 comments on Medscape from medical professionals. Not a single medical professional who commented there could tell the difference between self-published junk science from a quack (that was torn to shreds on the Internet by numerous reviewers) vs a double blind RCT study done by a very respected researcher at a top institution published as the lead article in the top peer reviewed medical journal (and where absolutely nobody could explain how the results were erroneous).
While few doctors felt comfortable proactively broaching this drug, 100% of the doctors I asked would discuss it with patients if the patient brings it up. That’s the key! The doctor will not proactively “push” a new treatment on a patient until he/she is 99.9% sure it works (99% isn’t good enough). Your doctor can then write a prescription which you can fill instantly at a pharmacy. The cost of the drug? About $13
Nearly 100% of the physicians I asked (including one of the top coronavirus experts in the world) who are familiar with the entire body of evidence supporting the drug would take this drug themselves if they got sick and couldn’t get the monoclonal antibody (or an antiviral such as camostat).
In short, the position of the medical community is, “yes, this absolutely could save the lives of hundreds of thousands of people, and sure, we’d take it ourselves if we get sick, but we aren’t even going to let people know about it until we are absolutely sure it works because that would be irresponsible.” I would argue the opposite. It should be OK for doctors to objectively present you with the facts on something that could save your life and let you decide. What are we thinking? If people could see what’s really going on in hospitals today, there would be no more complacency.
Hydroxychloroquine had nowhere near this kind of evidence (it was not an RCT, they excluded data points they didn’t like, the doctor wasn’t well respected, it was self-published, and the paper was torn to shreds by readers) yet it got massive press and widespread adoption by the medical community. The present case couldn’t be more different! This is an RCT done by a world-class researcher at a world-class institution, it was published in the world’s top peer reviewed journal, the PI has done 10 studies and none have been overturned, there has confirmatory observational data that is incredibly compelling, nobody can “explain away” the outcome, and it gets nearly zero mainstream press attention. Does that make sense? Of course not! What is happening here is that doctors hear about this study and because it worked so well, they instinctively react with disbelief (i.e., “that’s too big an effect to be real”), and instinctively equate this with the HCQ study, and conclude, “more studies need to be undertaken before raising hopes of the public.”
So there you have it. The solution to immediately easing the burden of this pandemic on both patients and hospitals is hiding in plain sight. It has the potential to reduce the hospitalization rate by 85% or more (which implies a commensurate reduction in the fatality rate). All you have to do is ask!
And if you can’t get fluvoxamine, you can likely achieve similar protection with doxazosin. With doxazosin, there is a clear mechanism of action and clear confirmation from observational studies. Doctors I spoke with knew of only one person in the US who died from COVID that was taking doxazosin. And if you have high blood pressure, it’s a freebie because you address your high blood pressure and COVID with the same medicine. The evidence is so compelling that the people who understand the drug the best (who are arguably some of the smartest people in the world) have stockpiled it for use when they get sick. The medical community is conservative and requires a trial. Unfortunately, John Hopkins is not allowing the trial to proceed and the most compelling observational data for doxazosin is not allowed to be published. So why then should you believe me? You don’t have to. Just read the authors list of the doxazosin paper, in particular Bert Vogelstein who came up with the idea. As Wikipedia notes, Vogelstein’s research papers have been cited over 420,000 times, more often than those of any other scientist, in any discipline, in recorded history. You really can’t beat that. And then you just have to trust me that I’m telling you the truth about the data they can’t publish.
Here’s the key point:
The reason our hospitalization rate is so out of control is that the medical establishment requires phase 3 RCTs to change clinical practice. This is impractical in a pandemic. Where we don’t have the answers patients need, we owe it to patients to put all the options on the table, discuss the pros/cons, and allow patients to decide which option to take.
I know of one doctor treating over 200 cases in California. Currently, there is a 10% hospitalization rate on people who refuse the fluvoxamine, 0% hospitalization rate on those who take the doctor’s advice. He will submit it to JAMA, but the medical community will also ignore this evidence because it was not in a clinical trial. That is what they are trained to do. How many more people must die before they believe the evidence? The answer, sadly, is that there is no limit; it as many as it takes before a phase 3 trial is completed. A Wall St. Journal op-ed published November 24 complained about the exact same thing: doctors are ignoring the high quality evidence in plain sight and it is costing lives.
Today, US hospitals will not allow doctors to ask patients if they want to try fluvoxamine outside of a clinical trial and the hospitals themselves are too busy to open a clinical trial. Catch-22! We need to allow doctors to use their best judgment here in a pandemic. But we don’t. The ER docs would lose their jobs if they did that. There is now an in-patient fluvoxamine trial in Hungary, but nowhere else in the world.
To provide the additional evidence needed by doctors, CETF is funding the follow-on phase 3 trial of fluvoxamine, but those results won’t be available for at least two months. This has the potential to be game changing in reducing the amount of time patients need to spend in the hospital.
In summary, the solution to COVID is clearly hiding in plain sight, but patients have to ask for it.
NOTE FOR PREPARING FOR NEXT PANDEMIC
It would be really nice if today we allocate a fund of $200M to be used to test repurposed drugs in an already setup multi-center outpatient adaptive clinical trial network. We screwed up big time on this one, and it’s no excuse for not putting this in place for the next one which could happen at any time. We shouldn’t be complacent about this. All of our arguments about whether a particular drug works or not could have been objectively and quickly settled. Camostat would have been tested 11 months ago, etc. Contrast this to today where we are still debating whether or not HCQ works.
References
[1] We have an inexpensive, FDA-approved drug that can reduce the hospitalization and death rate from COVID-19 by 85% or more… but only if we start using it now explains the 85% calculation, the mechanism of action, and lists 21 arguments for why everyone should consider this.
Steve Kirsch is an entrepreneur and medical philanthropist based in Silicon Valley. He started the Covid-19 Early Treatment Fund (www.treatearly.org) in April. The fund was one of the sponsors of the fluvoxamine outpatient clinical trial and is sponsoring the follow on Phase 3 trial for fluvoxamine.